Lithium nitride is the only stable alkali metal nitride
Overview of Lithium Nitride Li3N Powder
Lithium nitride is a metallic nitrogen compound with the molecular formula Li3N. It is the only stable alkali metal nitride. It is a purple or red crystalline solid, with a high melting point and a light green luster in reflected light and ruby color in transmitted light. Exposure to the air over a long period of time will eventually result in lithium carbonate. The chemistry of basic metal nitrides is extremely limited, and only lithium nitride is stable and easily prepared among binary compounds (sodium nitrite and potassium nitrite are prepared only under more extreme conditions).
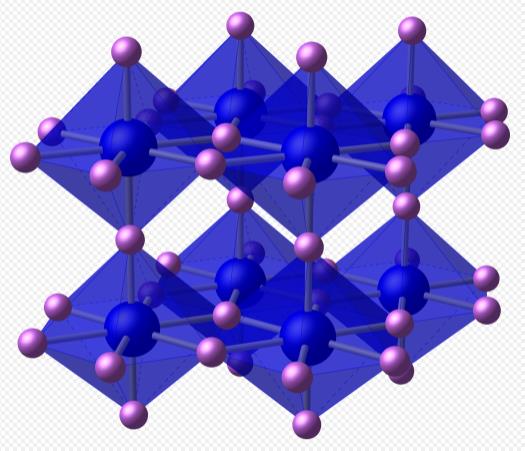
Lithium nitride belongs to the hexagonal crystal system. There are lithium and nitrogen layers composed of lithium and nitrogen atoms in lithium nitride crystal. The lithium atoms are arranged like carbon atoms in a graphite crystal, with nitrogen atoms at the center of a hexagon of lithium atoms. There is a lithium layer between the lithium and nitrogen layers. Because the ratio of lithium to nitrogen in the lithium and nitrogen layers is 2:1, that is, Li2N, which does not conform to the stoichiometric formula Li3N, there is a lithium layer between every two lithium and nitrogen layers. In the crystal cells of lithium nitride, the distance between Li-N is 213pm, which is close to the sum of the ionic radii of lithium-ion and nitrogen negative ions. The distance between each lithium and nitrogen layer and the adjacent lithium layer is 194pm, which indicates that lithium nitride is an ionic compound.
At room temperature, lithium metal exposed to air can partially form lithium nitride, lithium in the nitrogen flow to form lithium nitride, 10 -- 15 times faster than in the air, then all lithium into lithium nitride. In contrast, other alkali metals are difficult to form nitrides. Sodium nitrite, for example, can only be prepared by depositing atomic beams on sapphire at low temperatures and decompose with slight heat. Lithium nitride is easily hydrolyzed to produce lithium hydroxide and ammonia gas, especially fine powder lithium nitride, heated in the air can occur violent combustion. Therefore, lithium nitride must be handled in an inert atmosphere (e.g., nitrogen). Can be used as nitriding agent, reductant in organic reaction and source of nitrogen in inorganic reaction.
Lithium nitride has been discovered since the late 19th century and is easily prepared by combining elements. The study of the reaction of lithium nitride with hydrogen gas began in the early 20th century. Daft and Miklauz discovered that lithium nitride reacts with hydrogen gas at 220-250℃ to form a substance composed of "Li3NH4". They continue to heat this substance at higher temperatures (> >;700℃) into "Li3NH2" and hydrogen.
Applications of Lithium Nitride Li3N Powder
Lithium nitride is prepared by direct reaction of elemental nitrogen and lithium, usually by burning lithium in pure nitrogen. This method is the most commonly used method for preparing lithium nitride, both in the laboratory and in the industry. Alternatively, nitrogen can be injected into liquid sodium dissolved in lithium metal, which produces a more pure lithium nitride. There are many applications of lithium nitride, as follows:
1. Solid electrolyte
Lithium nitride is a fast ionic conductor and its electrical conductivity is higher than other inorganic lithium salts. Many studies have been conducted on the application of lithium nitride as a solid electrode and cathode material for batteries.
2. Fast ion conductor
A series of lithium fast-ion conductors were prepared based on lithium nitride. As a fast ion conductor material, it should have higher decomposition voltage, lower electronic conductivity, higher ionic conductivity, and better chemical stability.
3. Preparation of boron nitride from lithium nitride
Lithium nitride (Lin3) is a catalyst for the synthesis of (boron nitride) CBN under high temperature and pressure. Lithium nitride catalyzes the conversion of Bn to CBN at high temperature and high pressure, and the reaction of B4C with NH4A at atmospheric pressure and high temperature. It can also be used as a nitrogen source in the solvothermal reaction with Bbr3 to produce Bn and CBn.In addition to being used as a solid electrolyte, lithium nitride is an effective catalyst for the conversion of hexagonal boron nitride to cubic boron nitride.
4. Lithium difluorosulfimide salt was prepared by using lithium nitride.
Lithium difluorosulfimide (LIN (SO2F)2, hereinafter referred to as LIFSI) is an electrolyte substance with wide application prospects. LiFSI has suitable conductivity, high thermal stability, and electrochemical stability
5. Using lithium nitride to prepare cubic boron nitride
Preparation of lithium boron nitride (Li3BN2).Li3BN2 is an important catalyst for the synthesis of cubic boron nitride superhard material and has been used in the industrial production of cubic boron nitride synthesis. The hardness of cubic boron nitride is lower than that of the diamond, but diamond can not be used for grinding ferrous metal, so cubic boron nitride is the best grinding material for ferrous metal and its alloy at present.
Lithium Nitride Li3N Powder Price
Lithium Nitride Li3N Powder price will vary randomly with the production cost, transportation cost, international situation, exchange rate, and market supply and demand of Lithium Nitride Li3N Powder. Tanki New Materials Co.Ltd Aims to help All industries and Chemical Wholesalers to find high-quality, cheap price Nanomaterials and chemicals by providing turn-key customize manufacturing services. If you are looking for Lithium Nitride Li3N Powder, please feel free to send an inquiry for the latest price.
Lithium Nitride Li3N Powder Supplier
As a global Lithium Nitride Li3N Powder supplier, Tanki New Materials Co.Ltd has rich experiences in the properties, applications, and cost-effective manufacturing of advanced and engineered materials. The company has successfully developed a series of powder materials (including oxides, carbides, nitrides, single metal, etc.), high-purity targets, functional ceramics, and structural devices, OEM service is available.
More information about Lithium Nitride Li3N Powder
Lithium Nitride Li3N Properties (Theoretical) | |
Compound Formula | Li3N |
Molecular Weight | 36.8456 |
Appearance | Powder |
Melting Point | N/A |
Boiling Point | N/A |
Density | 1.3 g/cm3 |
Solubility in H2O | N/A |
Exact Mass | 37.0667 |
Monoisotopic Mass | 37.0667 |
Charge | 2 |
Lithium Nitride Li3N Health & Safety Information | |
Signal Word | Danger |
Hazard Statements | H260-H314 |
Hazard Codes | F,C |
Risk Codes | 11-14-29-34 |
Safety Statements | 16-22-26-27-36/37/39-45 |
RTECS Number | N/A |
Transport Information | UN 2806 4.3/PG 1 |
WGK Germany | 3 |
MSDS / SDS | Request MSDS / SDS |
留言
發佈留言